translational & preclinical drug development
Empowering Research for Future therapies
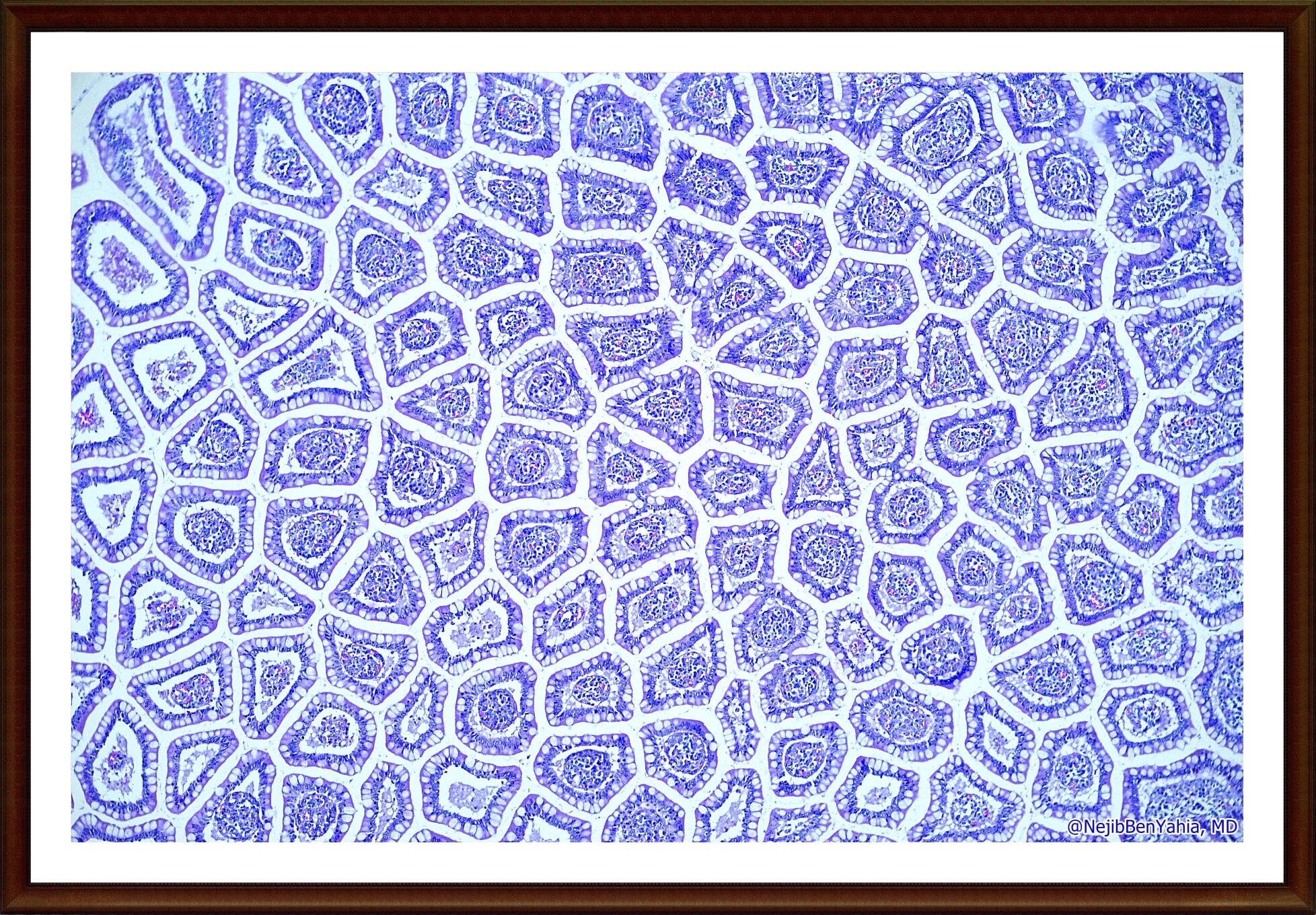
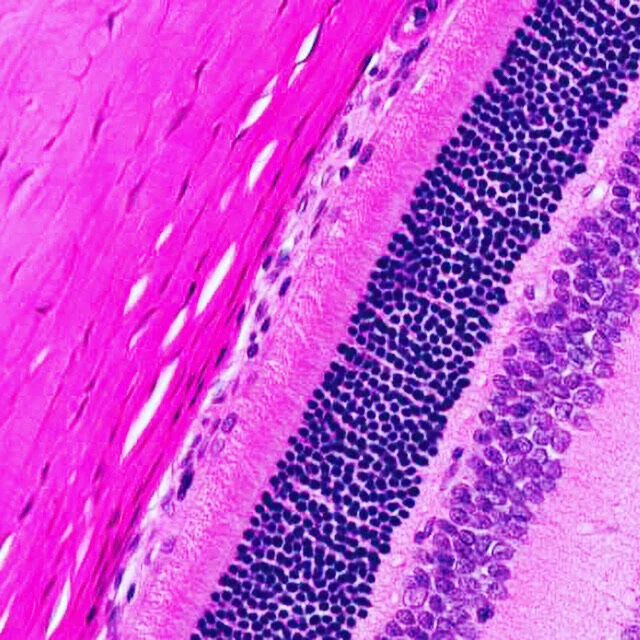
ANATOMO-PATHOLOGY
gross pathology & histology
Necropsy Specimens will only achieve their objectives if biopsy/full sampling, collection, fixation & transportation are conducted according to Specific guidelines and/or standard operating procedures.
Understand gross technics in necropsy rooms
manage & control your handling & fixation technics
trimming Guidelines for safety studies
State-of-art Histology procedures
trimming, Embedding & Cutting procedures
Revision & update Histology protocols
Expertise
Oral, Dermal, infusion, inhalation, carcinogenicity
Ocular Pathology
microscopic toxicological pathology
Preclinical R&D programs including adequate IND submissions represent a regulatory-driven prerequisite before Authorising the administration of a new drug or implantation of a new medical device. The role of the Toxicological Pathologist is to act as a preclinical expert: He/She diagnoses, interprets and deliver written risk assessments on any organ/tissue change related directly or indirectly to the administration of the new drug. the final pathology report represents the end-result of the in vivo experiments and will be part of the IND submission.
DIGITAL PATHOLOGY
Digital pathology has been widely discussed since several years in Societies of Surgical Pathologists. Recently, it's use has been widely increased in additional fields such as in Academia for educational purposes, but also in Non-Clinical drug development research.
In 2017, the FDA approved for the first time the Whole Slide Imaging Systems for digital pathology that allows the interpretation of digital surgical pathology slides prepared from biopsied tissues. In this non-binding guidance FA describes the details about the technical performance of WSI. Additional Peer-Reviewed publications are coming soon and will be announced to all followers of digital pathology.
Since, 2009 I have been trained within a digital pathology Environment and in 2019, I obtained nSH/DPA certification in digital pathology. Special blog posts are published on a regular base including Podcasts giving you the latest updates about digital pathology within toxicological pathology.
Feel free to send me your questions about digital pathology
Double-board certified toxicology
20-years expertise in non clinical drug development
global roles & expertise fields of a Board-certified Toxicologist
Designs & Interpret nonclinical safety & efficacy studies
solicits bids to evaluate and select the appropriate contract research Organisations
manages & monitors preclinical and nonclinical studies
serves on project teams, expert panels, & scientific advisory boards
assists in new project development an existing product Defence
reviews & writes expert reports, white papers, study reports, product Labelling, safety data sheets; investigator brochures
acts as a liaison between sponsors and regulatory authorities
provides gap analysis & addresses needs & assessments for existing programs
renders subject matter expertise during due diligence activities
Assists financial forms in evaluation of specific data for small & large research institutes and pharmaceuticals
types of toxicologic programs
Depending on the type of Drug and the stage of development, the translational toxicologist can support you to make the appropriate strategic decisions in order to move forward your drug development process.
tox programs are designed in function of the drug molecule, type of molecule, the stage of development an/or specific regulatory requests.
Special Toxicologic & Pathology Programs
In addition to the regular tox programs it’s always nice to distinguish special expertise niches, as mentioned below.
Feel Free to click on these special sections for additional comprehensive Knowledge.
Special ToxProgram: Tobacco Products (Coming soon)
TRANSLATIONAL WELFARE
3R’s also called research animal replacement, refinement, and reduction of test strategy demand that key personnel from industry and FDA who are involved in the submission and the review of safety data Consider a full integration of clinical, toxicological, and veterinary methodology.
a certified laboratory animal expert (art.9) is trained
to monitor the execution of approved Experimental procedures & projects
to ensure compliance with the regulations and AAALAC requirements.
is actively involved in health and welfare of laboratory animals
Provides scientific support, education & training of all people involved in animal experimentation.
He/She audits CRO’s and academic research laboratories, laboratory animal breeders and other suppliers with excellence.
GLP: QUALITY ASSURANCE
compliance 21 cfr part 11
Title 21 CFR Part 11 is the part of Title 21 of the code of federal regulations that establishes the United States Food and drug Administration (FDA) regulations on electronic records and electronic signatures. Part 11 defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable and equivalent to paper records. Practically Part 11 applies to pharmaceutical companies, medical devices manufacturers, biotechnology companies, sometimes even GLP departments in academic settings.
Good Laboratory Practices
in the experimental or translational research area, GLP is a quality system of management controls for laboratories and Organisations who need to ensure the uniformity, consistency, reliability, reproducibility, quality and integrity of products in development for human and animal health through non-clinical safety tests; varying from physiochemical Properties through acute to chronic toxicity tests.
roles & responsibilities of a Toxicologic Pathologist in qA
understanding the GLP-workflows from accredited Good Laboratory Practice laboratories
Understanding, reviewing & Implementing standard operating procedures (SOP’s)
auditing nonclinical safety studies, histology & pathology procedures
providing mock inspections in order to prepare for official regulatory authorities
revisions of raw data, incl. necropsy procedures
corrective actions related to animal health procedures
Need some examples: check your QA blog spot !